Plants produce an enzyme called acetolactate synthase (ALS) that catalyzes the first step in the production of the essential amino acids valine, leucine, and isoleucine, which are used to build other proteins. By inhibiting the enzyme, you can stop production, which will kill the plant. This can be done with a variety of weed removers that bind to the enzyme and prevent its function. If you spread these weed removers over a rice field, you will kill all weeds, but also the rice plants. Thus, it would be very useful to make rice plants that were resistant to the weed remover.
A Chinese research team succeeded in creating genetically modified resistant rice plants, using CRISPR/Cas9 and HDR to make a gene knock-in in the ALS gene by replacing part of the sequence. The rice plants were fed a plasmid containing the Cas9 gene, a DNA template, and two pieces of sgRNA. The two pieces of sgRNA were used to carve out a sequence in the middle of the ALS gene, and this DNA sequence was replaced with the DNA template by HDR. This DNA template was almost identical to the sequence of the wild type, except that it contained some specific mutations. These mutations meant that the acetolactate synthase produced was not susceptible to binding by the weed remover, thus preventing an inhibition of the production of the essential amino acids. Thus, it is a gain-of-function mutation due to a gene knock-in.
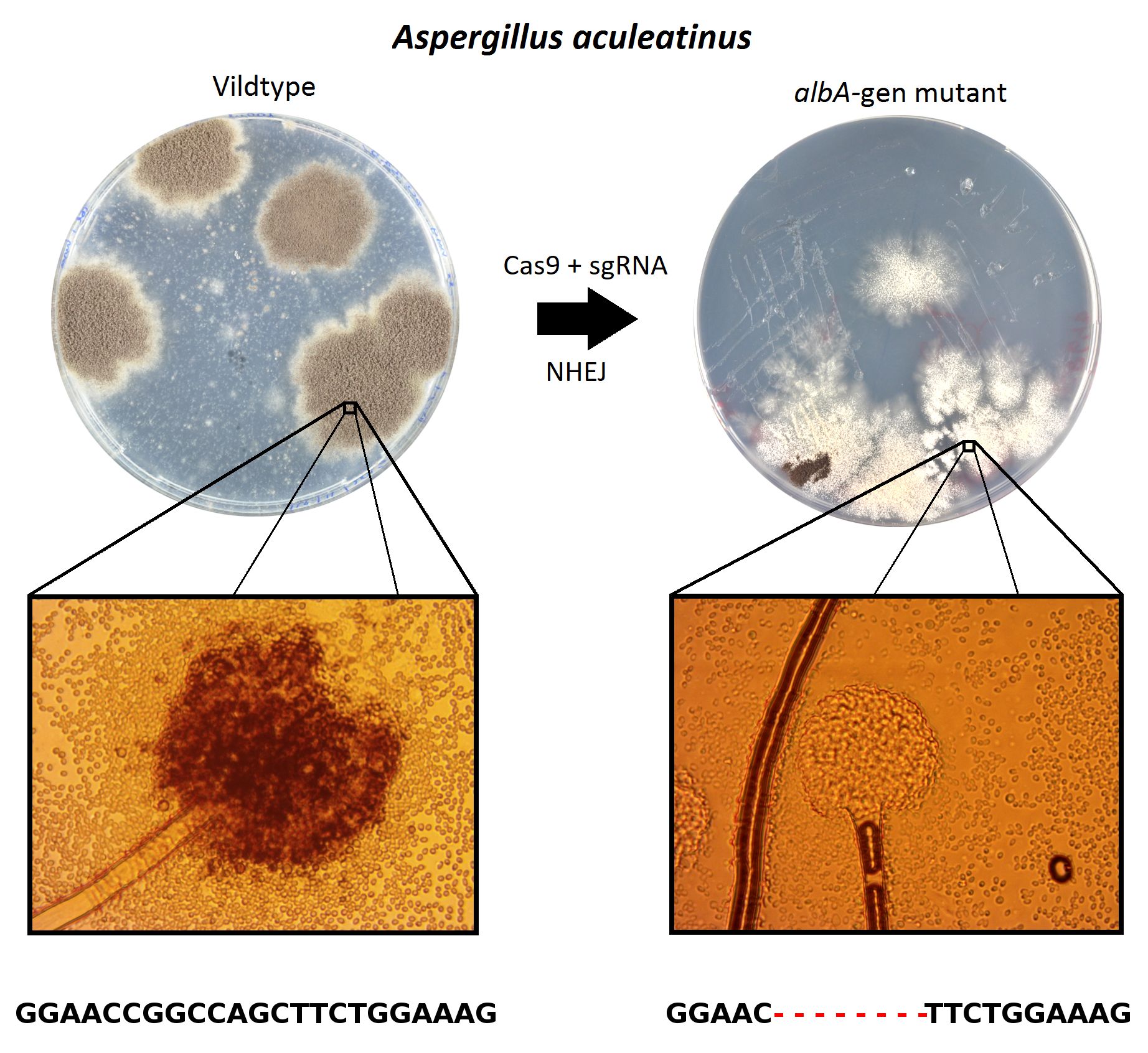
This genetic modification resulted in viable resistant rice plants, but fault mutations were also observed due to inappropriate repair of NHEJ. Using the rice plants that were modified correctly, one would be able to plant a rice field of resistant plants. In this rice field, you will be able to remove all weeds with the weed remover, but let the rice plants thrive. In Figure 13, the experimental results can be seen, where it is clear that only the genetically modified plant thrives despite the presence of weed remover.