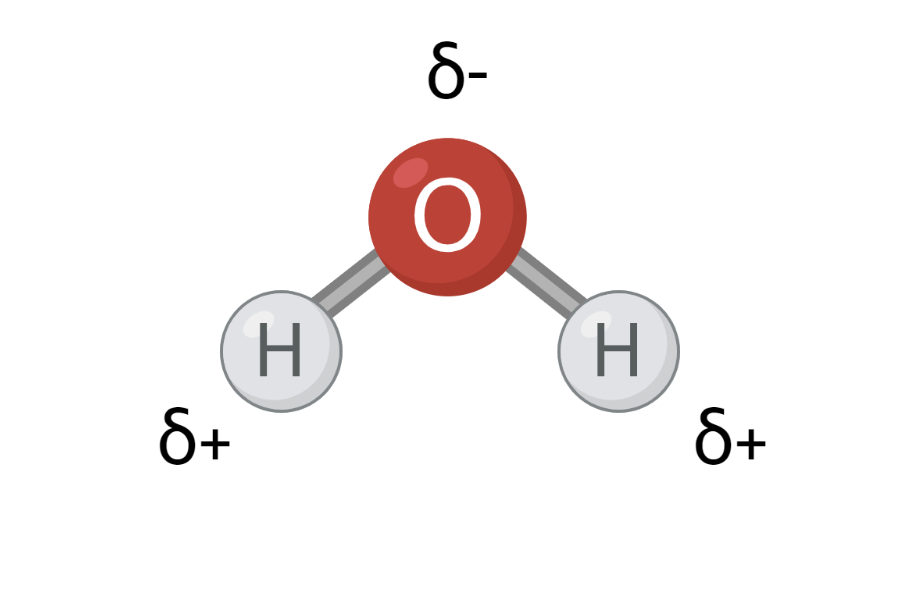
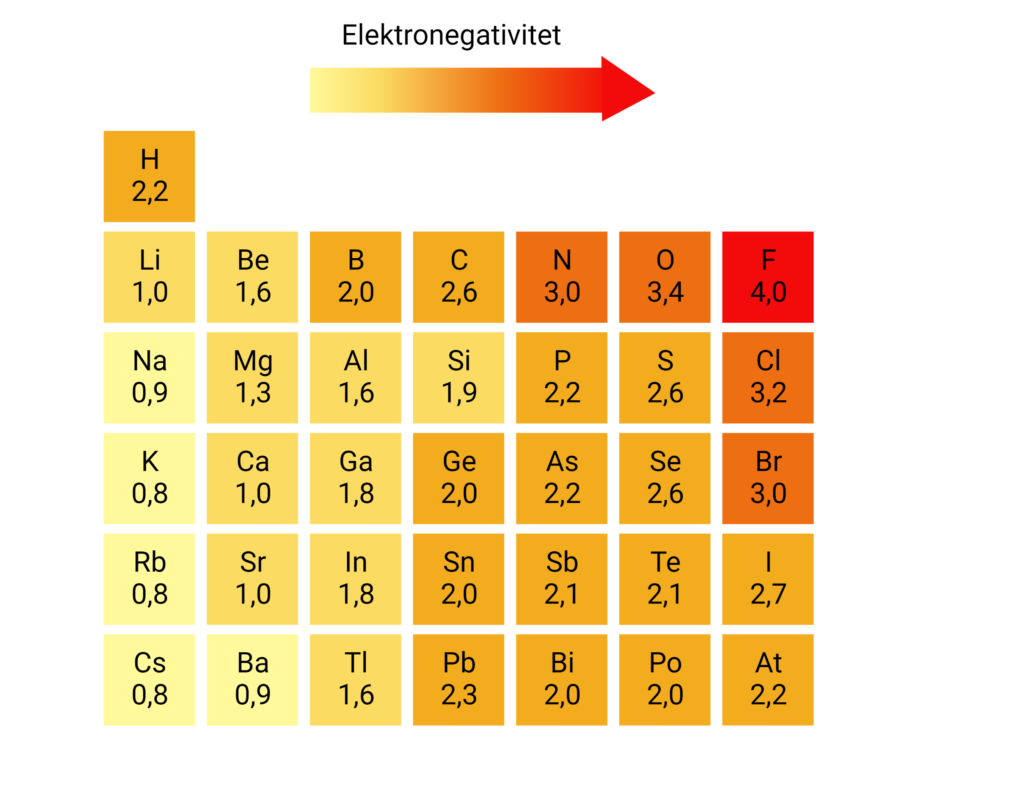
Polarity is a partial shift in the charge of a molecule. In molecules, the atoms are bonded together by sharing electrons with each other, but the electrons are not always distributed equally between the atoms. Some atoms pull more on the electrons than others, and this is how polarity occurs. In a polar molecule, one part of the molecule is positively charged, while another part of the molecule is negatively charged. You can see an example of a polar molecule in Figure 1. The ability of an atom to pull in the electrons is called electronegativity. In Figure 2 you can see the electronegativity of a number of different atoms.
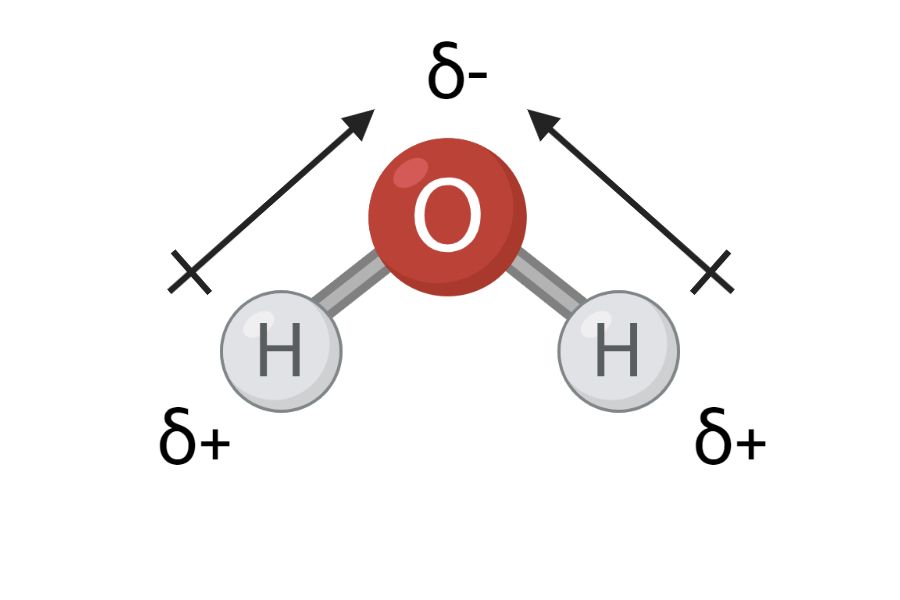
Atoms themselves have no superior charge because they have equal numbers of electrons and protons. However, when they enter into bonds with each other, they can get a small charge. This is because the electrons in the bond may be closer to one atom than the other. Thereby, there are relatively fewer or relatively more electrons compared to the company. protons around each atom.
In Figure 2, you can see that oxygen has an electronegativity of 3.4, and hydrogen has an electronegativity of 2.2. This means that oxygen pulls more in the electrons than hydrogen in an O-H bond. The electrons in the O-H bond will therefore be closer to oxygen than hydrogen – also called a charge shift. Electrons are negatively charged, and when they are closer to oxygen, the oxygen atom acquires a partially negative charge. At the same time, the electrons are located a little further away from hydrogen, and therefore the hydrogen atom receives a partially positive charge. The partial charge is indicated by a small delta, δ, above each atom and either a minus (-) or plus (+), as can be seen in Figure 3.
Electronegativity difference
To find out if a molecule is polar, one can calculate the electronegativity difference of all the bonds in the molecule. Taking water, H2O, as an example, calculate the electronegativity difference in the O-H bonds. By reading the values in Figure 2, one can calculate the electronegativity difference to 3.4-2.2=1.2 in each O-H bond. The greater the electronegativity difference, the greater the charge displacement and the more polar the bond. In Table 1 you can see the correlation between electronegativity difference and polarity. An O-H bond is thus polar.
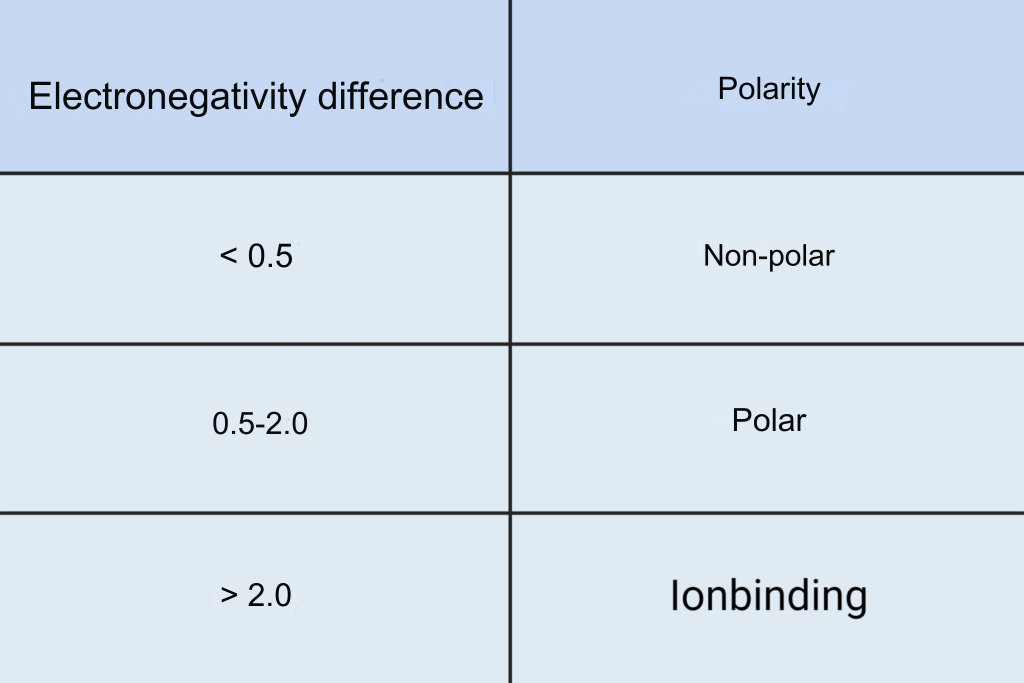
However, it is not enough to look only at the electronegativity difference of all the bonds in a molecule to determine whether it is polar.
Geometry affects the polarity of the molecule
You can only assess whether a molecule is polar by also looking at its geometry. The spatial structure of the molecule determines its overall charge. In Figure 4, you can see two examples of molecules with polar bonds: carbon dioxide and water. Earlier, you learned that the O-H bonds in water are polar. The C=O bonds in carbon dioxide are also polar because they have an electronegativity difference of 3.4-2.6=0.8. Despite the polar bonds, carbon dioxide is not a polar molecule. The atoms in carbon dioxide lie on a completely straight line, which means that there is 180o between the C=O bonds. The oxygen atoms therefore pull the electrons equally in opposite directions. This causes the two charge shifts from each C=O bond to cancel each other out. This means that the overall charge of the molecule is 0, and carbon dioxide is nonpolar. In water, the angle between the O-H bonds is 109o. Water is therefore a polar molecule.

The 1:4 Rule
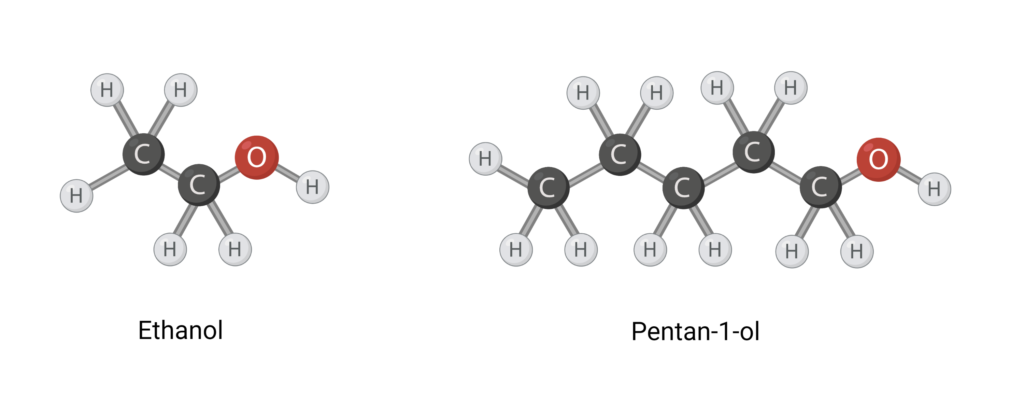
It is rare for there to be only one kind of bond in a molecule, as is the case for carbon dioxide and water. Therefore, when looking at the polarity of a larger molecule, one can look at the number of polar bonds in relation to the number of nonpolar bonds. If you work with organic compounds with carbon atoms, a rule of thumb is that it takes four carbon atoms with nonpolar groups to offset one polar group. Organic compounds often contain many C-H bonds. They are nonpolar, since the electronegativity difference is 2.6-2.2=0.4. Ethanol and pentan-1-ol in Figure 5 are two examples of organic compounds. Ethanol contains one polar O-H bond and two carbon atoms with nonpolar C-H bonds. Using the 1:4 rule of thumb, ethanol can therefore be considered a polar molecule. Pentan-1-ol, on the other hand, is an unpolar molecule. It contains five carbon atoms with C-H bonds that outweigh the polar O-H bond.
Hydrophobic and hydrophilic molecules
Hydrophobic and hydrophilic is another way to describe polarity. When we talk about hydrophilicity, we are talking about the ability of molecules to mix with water. Molecules prefer to mix with other molecules similar to themselves. Water, as already mentioned, is a polar molecule. Other polar molecules mix well with water, which is why they are called hydrophilic. Bipolar molecules do not mix very well with water, which is why they are called hydrophobic. Hydrophobic also means water resistant, and hydrophilic means water-loving. As a rule of thumb, you can think of a phobia as when you are afraid of something. Hydrophobic molecules are “afraid” of water, and therefore prefer not to mix with water.
An everyday example
Oil is a nonpolar molecule that contains long chains of carbon atoms with C-H bonds. It does not mix very well with water, which is polar. If you try to mix oil and water, the oil will accumulate into droplets on top of the water. It does this to reduce contact with the water. You can therefore also call oil hydrophobic.
« Back to Glossary Index
